Literally translated the word Photovoltaic means light-electricity. Photo is derived from the Greek word for light and volt in honor of electricity pioneer Alessandro Volta.
To understand how a solar cells works, we must first take the time to understand the properties of the materials used in the construction of a solar cell. There are different types of solar cells, we will not examine all so of them. Instead I will explain what is considered the primary type solar cell that is constructed from a single crystal of silicon semiconductor.
Silicon, chemical symbol Si, is an abundant material on Earth. The Earths crust is comprised of 25.7% silicon in its various forms. Silicon is may be found in sand, clay, granite, quartz, glass, cement and ceramics. It is the second most abundant element found on earth, after oxygen.
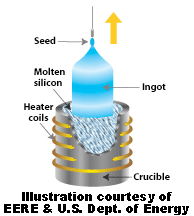
To be useful in electronics and for generating electrical power from solar energy, silicon must be highly purified (99.9999 % ). To purify silicon to such a high degree requires special processing. One purification method is the Czochralski process. In it, a seed crystal is dipped into a crucible of molten silicon and withdrawn slowly, pulling out a cylindrical single crystal out of the molten silicon that had crystallized on the seed. The cylindrical crystal is called a Boule. The Boule can be cut up with a diamond saw into slices (called wafers) and is used to make electrical components.
Pure silicon does not conduct electricity; in fact it’s an insulator. Each silicon atom has four electrons in its outer shell. Each atom fills its outer electron shell to eight electrons by sharing its four electrons with four other silicon atoms. The atoms form into a tight crystalline structure where all the electrons are held strongly in place. Without any free electrons to act as charge carriers pure silicon a good insulator.
What makes silicon so useful in electronics is that by adding a small amount of impurities to the silicon while it is being manufactured alters its electrical properties in a very useful way. The impurities are called dopants in the industry, and the process of adding the impurities is called doping. The amount of material used in doping silicon is on the order of one part in 100 million. There is so little dopant atoms introduced into the silicon that the silicon crystal lattice forms properly.

